Abstract
A subset of patients with chronic bleeding remain undiagnosed even after extensive diagnostic evaluation are labeled as "bleeding of unknown cause" (BUC). The key barrier to treating these patients is that they have a clinical bleeding tendency in the presence of normal diagnostic tests, and optimal methods for monitoring and treating patients with BUC remain unknown. While patients with BUC have symptoms of a primary hemostatic disorder, there is no diagnostic test or biomarker that can accurately identify which patients are at risk for bleeding such as those with mild Von Willebrand Disease (VWD) which comprise a broad spectrum of patients with varying degrees of bleeding. In order to fill this diagnostic gap in disorders of primary hemostasis, there is a clinical need for more assays of platelet function.
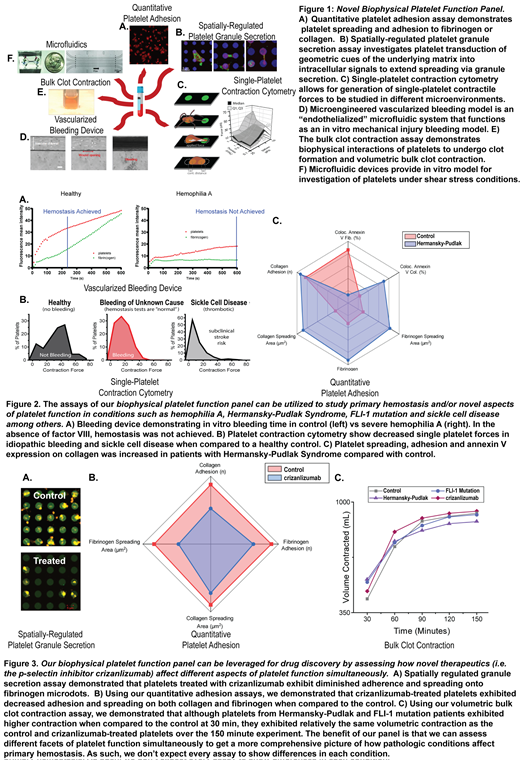
To that end, we have engineered multiple new biophysical assays to assess disorders of primary hemostasis and apply this panel of platelet function testing to potentially define new bleeding disorders, characterize platelet phenotypes in patients with BUC, and refine the definition of mild VWD. Our panel of platelet function tests (Fig 1) collectively enables us to simultaneously assess different facets of primary hemostasis from the microscopic level of single-platelet physiology to hemostatic plug formation, thereby capturing various aspects of platelet function with a single blood sample. Our platelet function panel ranges from platelet adhesion and bulk clot contraction assays to spatially-regulated platelet granule secretion assay, single-platelet contraction cytometry, microfluidics, and a microengineered vascularized bleeding model. As such, we are leveraging these biophysical assays to correlate platelet function with bleeding phenotype severity and establish the dynamic range of this diagnostic panel. We have now established that our assays can be utilized to study blood samples from patients with disorders including hemophilia A, Hermansky-Pudlak Syndrome, FLI-1 mutation, and sickle cell disease among others (Fig 2), demonstrating the clinical utility of our platelet function panel.
Our panel can also be used to assess the effects of novel therapeutics on different aspects of platelet function simultaneously. To investigate how crizanlizumab (p-selectin inhibitor) affects hemostatic plug formation, healthy human blood was treated with crizanlizumab. Platelet α-granule secretion enables exposure of P-selectin, and with crizanlizumab we observed restricted platelet filopodial extension and diminished α-granule exocytosis, and an overall decrease in adhered platelets to the fibrinogen micropattern (Fig 3A). The adhesion assay demonstrated a decrease in spreading and adhesion of platelets to collagen and fibrinogen with treatment (Fig 3B). Using the bleeding model, hemostasis was achieved within the normal established range and platelets contracted normally. This suggests that p-selectin has a limited role in the setting of minor injury. Utilizing the bulk contraction assay, we exhibited increased contraction early in clot formation, however over time the treated platelets contracted similarly to the control (Fig 3C). Interestingly, the effect of crizanlizumab-induced restriction of filopodial extension did not correlate with impaired bulk clot contraction or time to form hemostatic plug. Our work suggests crizanlizumab affects platelet spreading at the single-cell level but does not impair platelet function in achieving primary hemostasis at the whole blood level.
Here we demonstrate the translational utility of our platelet function panel in providing a deeper understanding of platelet biophysics as it relates to hematologic conditions, with implications for investigation to include pharmaceutical applications. The versatility of this novel panel in capturing platelet function from single-platelet contraction to providing in vitro models with the bleeding device provides multiple dimensions to platelet investigation for primary hemostatic disorders and BUC that have not yet been elucidated. Ongoing research is being conducted using our comprehensive platelet function panel to investigate platelet properties in BUC and mild VWD and correlate these biophysics with bleeding phenotypes. Using this approach we aim to provide novel diagnostic testing with clinical relevance for disorders that have been incompletely characterized until now.
Meeks: National Institutes of Health: Research Funding; Hemophilia of Georgia: Research Funding; National Hemophilia Foundation: Research Funding; Spark Therapeutics: Consultancy; Sangamo Therapeutics: Consultancy; Pfizer: Consultancy; Sanofi: Consultancy; CSL Behring: Consultancy; Genentech: Consultancy; Takeda: Consultancy. Lam: Sanguina, Inc.: Current holder of individual stocks in a privately-held company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal